
Electroplating is a primary surface engineering process that is widely utilized in the storage & industrial manufacturing. An electric current is used to electro deposit a metallic coating onto a conductive substrate. The principal objective of plating is to improve surface attributes like hardness, electrical conductivity, corrosion resistance, & aesthetic appeal. Increasing component longevity, enhancing their mechanical properties, and decreasing operating downtimes in manufacturing settings are all rendered possible by this method of improvement.
In a standard industrial environment, electroplating ensures consistent coating distribution on components with sophisticated geometries, such as large storage containers & racks, machine tools, fasteners, including automotive components. It is a vital approach for businesses that operate in demanding conditions where failure of equipment may result from surface deterioration caused by prolonged contact with chemicals, moisture, or abrasion.
Electroplating's history dates to quite prehistoric times. Although they lacked the modern scientific basis, early versions of metal deposition & gilding were used in Mesopotamia and Egypt. The real triumph came with the development of electrochemistry in the first half of the nineteenth century, particularly after the revolutionary electrical circuit discoveries achieved by Luigi Galvani & Alessandro Volta.
With the invention of improved electrolytes, rectifiers, and procedure automation, electroplating became crucial for heavy industry by the beginning of the twentieth century. Nowadays, electroplating is a digitally controlled, precision-driven process which must be performed to produce robust and high-performing components for the energy, storage, automotive, as well as aerospace industries.
In essence, electroplating is a reduction process that is electrochemical and controlled by Faraday's Laws of Electrolysis. The process takes place in an electrolytic cell that consists of four important parts:
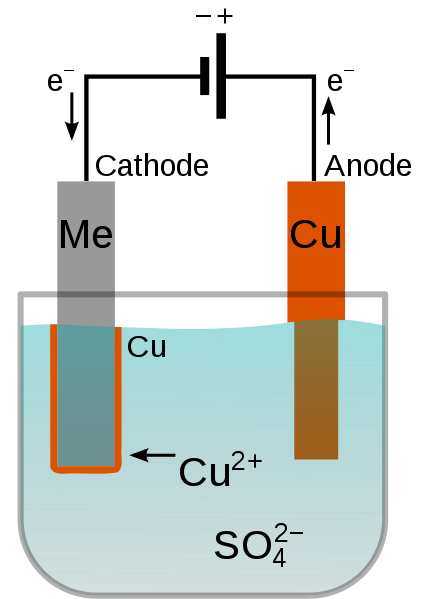
Positively charged metal ions in the electrolyte, such as Ni2+ & Zn2+, move into the negatively charged cathode when DC is applied. When these charged particles come into contact with the substrate, they initiate reduction, which results in the emergence of a solid metallic layer. For the purpose of keeping the electrolyte balanced, the anode metal dissolves concurrently & adds new metal ions.
In industrial purposes electroplating operations, it becomes crucial to comprehend & regulate these factors, particularly when working with large-scale batch production or high-tolerance parts. The performance of the product in both manufacturing and storage applications may be greatly affected by changes in these variables, which may result in problems like uneven coating, pitting, or breakdown.
One important element which impacts the final product's mechanical, chemical, & electrical performance is its selection of electroplating metals. In commercial production and storage settings, selecting the kind of metal has a direct impact on functionality, durability, & regulatory compliance since every material imparts unique qualities to what it coatings.

In heavy industry, nickel serves as one of the most commonly used and adaptable plating metals. It offers moderate brightness & hardness in addition to an effective barrier against abrasion as well as corrosion. Whether a corrosion-resistant foundation coating is needed underneath ornamental or functional topcoats, nickel is often used in multi-layer systems. It is vital in operations requiring storage fixtures, gears, & equipment that endures exposure to chemicals or mechanical wear
A typical protective coating for steel parts is zinc. The primary objective is galvanic protection, that prolongs the life of the base metal by allowing the zinc to corrode preferential to the underlying substrate. Bolts, nuts, & racking systems are examples of these zinc-plated components that frequently appear in settings where contact with moisture or acidic substances is an issue. Chromate transformation coatings are often used to passivate zinc with the goal to enhance look & extend protection.
In electroplating, copper has two distinct applications. due to its superior conductivity & stickiness, it frequently serves as an undercoat in multilayer systems. Furthermore, it performs essential duties in the electronics manufacturing industry wherever effective current transmission is necessary. Heat exchangers, busbars, & printed circuit boards frequently involve electroplated copper.
In addition to its outstanding resistance to wear plus its superb polish, chromium is highly sought after. Industrial hard chrome plating is applied to spinning machinery, piston rods, as well as hydraulic cylinders to reduce friction & improve service life. Due to their greater cost, metals such as silver and gold are used less frequently, usually in aeronautical assembly, high-reliability electronic connectors, and essential medical parts wherein conductivity & corrosion resistance are essential.
The multi-stage, controlled electroplating process converts electrical & chemical energies into a durable, useful metallic coating. Process integrity is crucial in manufacturing environments because just one part failure could lead to system-wide inefficiencies or stoppage. Surfaces preparation, metal depositing, & post-treatment are the 3 main stages of the plating cycle.
Probably the most significant phase during the entire procedure is this particular one. For the base material & the newly applied metal layer to adhere, the surface has to be tidy and active. Any lingering impurities, such oil, oxides, or elements, might result in peeling, voids, or irregular thickness.
To get rid of organic residues & oils, the cleaning step usually starts with alkaline degreasing, which can frequently be helped by electrocleaning or ultrasonic agitating. Acid pickling comes next, in which oxides on the surface & scale are dissolved by concentrated mineral acids (for instance, sulfuric or hydrochloric acid).
In particular situations, activation treatments like reversed-current electrolysis or immersion in halide-based solvents are used to increase surface energy, particularly with titanium or stainless steel.

The substrate is submerged in an electrolytic bath that contains dissolved metal salts along with particular additions (levelers, brighteners, grain refiners) after it has been correctly prepared. The anode, which is usually composed of the plated metal or an inert element like titanium covered with platinum, finishes the circuit after the component is linked to the cathode terminal.
Cations made of metals (such as Ni²⁺, Zn²⁺, and Cu²⁺) travel across the fluid called electrolyte & diminish at the cathode surface when current from the source is supplied, producing a cohesive metallic layer. Many interrelated components affect the coating quality & deposition rate:
The closed-loop chemical-based control systems including automated rectifiers are employed in modern plating handles to constantly preserve these values.
In order to get rid of any leftover electrolyte after deposition, the coated portion is rinsed in deionized water. In an effort to prevent embrittlement while utilizing high-strength steels, parts may occasionally undergo thermal processing, such at 190–220°C, to get rid of hydrogen absorbed during plating.
Lastly, to enhance corrosion resistance or fulfill particular mechanical or esthetic needs, protecting conversion coatings, topcoats, or lubricants can be added. To ensure quality control in large quantities manufacturing, intra-line drying, inspection, & thickness testing (by employing coulometry or XRF) are integrated.
The electroplating process's adaptability accounts for its resilience. Electroplating is still significant today for modern manufacturing, from thick, resilient to wear overlays on industrial use shafts to nanometer-scale coverings on microchips.
There is no standard technique for electroplating. A wide variety of application methods, each adapted to certain situations, must be used because of the variety of industrial components that vary in size, geometry, substance, & end-use environment. Four basic plating techniques rack plating, barrel plating, brush plating, & pulse plated dominate nowadays in the manufacturing and storage sectors. A variety of variables, such as part geometry, surface area, fragility, manufacturing facilities volume, & required coating uniformity, affect the method of choice.
The preferred technique for fragile or big parts that need exact integrity of the surface & coating thickness is rack plating. For the purpose of guaranteeing perfect electrical contact as well as low mechanical stress during immersion, parts are connected to customized conductive racks or fittings. For items like car frames, storing cabinet enclosures, HVAC components, including structural machine parts, this method is excellent. If many finishes are required on the same part, rack plating enables selective masking while offering greater oversight over plating distribution.

Connectors, nuts, washers, and many other small, sturdy objects can be processed in huge quantities using barrel plating. A turning barrel with perforation that have been partially submerged in an electrolyte bath is filled with these parts. The electrolytes & current path remain in contact with all surfaces due to the tumbling action. The mass produce of electroplated hardware for shelving units, flexible storage systems, including machinery attaching kits takes extensive use of this process, notwithstanding knowing that it is not suitable for pieces with tight tolerances for dimension or essential finishes on the surface.

A lightweight and targeted electroplating method, brush plating is primarily employed for on-site component repair, refurbishment, and selective improvement of specific areas. An electrolyte-saturated brush or pad is used to apply to the object's surface after being linked to a power source. Since disassembly or immersion are not achievable, this method is often used in tooling repair, storage tank refurbishing, & aerospace maintenance. Brush plating provides consistency & eliminates the need for remaking the entire part, notwithstanding its size and rapidity limitations.

Instead of delivering current constantly, pulse electroplating provides it in regulated bursts. By the alteration of waveform shape & on/off timing, this method enables greater control over deposit characteristics. It is especially helpful to improve deposit adherence, decreasing internal tension, and generating dense, fine-grain coatings. In particular industries where traditional plating has failed to meet exacting quality requirements, like microelectronics & aircraft storage systems, pulse plating is growing increasingly common.

In an array of industrial production & storage applications, electroplating serves as a functional enabler. The method offers observable mechanical, electrical, as well as chemical advantages that go well beyond the way it looks and directly support system reliability, asset longevity, and upkeep effectiveness.
Components are frequently subjected to severe mechanical stress, heat, chemicals, or corrosive conditions in workplaces. By changing the surface to resist severe deterioration, electroplating improves the components' longevity.
To significantly boost wear resistance as well as lower the coefficient of friction, automobile manufacturers, for example, employ electroplating to coat shock absorbers, engine housings, piston rods, as well as drive shafts with nickel or hard chrome. Electrical contacts, fasteners, as well as structural assemblies in aerospace are treated with small coatings of cadmium or gold to offer conductivity & corrosion protection at high temperatures along with altitudes.
Gold and copper electroplating is crucial in the electronics other semiconductor manufacturing industry to create conductive channels in printed circuit boards (PCBs), connections, including microelectronic assemblies. Here, even coatings as thin as microns have to conform to stringent requirements in order to keep vital systems from malfunctioning.

Furthermore, electroplating has significance in circumstances like large-scale storage infrastructure, chemical containment, & inventory control. Pallet frames, modular racking systems, thus metal shelving units are often zinc-plated in order to prevent them from rusting in order to preserve their aesthetic appeal, especially for warehouses that have different humidity levels.
Internal lead or tin electroplating can be helpful for chemical storage tanks, especially those holding corrosive acids, solvents, or bases. This reduces the chance of contamination & produces a chemically inert shield that safeguards the tank's foundation from damage.
Conveyors, mechanical guides, & motor housings constitute some of the vital parts of warehouse automation systems that have been electroplated. Coatings on these components must be robust to abrasion, exposure to lubricants, and prolonged use.

In the industrial sector, electroplating offers numerous advantages of engineering & financial benefits.

Even with its advantages, electroplating has an assortment of both technical and legal issues, particularly when used in large-scale manufacturing processes.
The Coating Uniformity precise process control with jigging are necessary for guaranteeing a constant deposition on parts with intricate geometries or recessed portions (Faraday cage effect).
When Hydrogen embrittlement is electroplated with zinc or cadmium, strong steels may absorb hydrogen atoms, which may lead to internal breaking. Baking after platting is often required.
Surface flaws that influence both functioning & appearance include pinholes, roughness, as well as adhesion failures induced on by inadequate cleaning or bath contaminants.
Hazardous materials such heavy metals, cyanides, especially hexavalent chromium frequently turn up in electroplating baths. According to EPA or REACH (EU) regulations, effluent treatment facilities are required for preventing contaminating groundwater. given the dangers of chemical exposure, worker safety procedures (which include fume hoods, personal protective equipment, & ventilation) must be used.
Electroplating technologies are advancing quickly as businesses strive for increased automation, precision, & sustainability. Amongst the major new trends are:
A shift away from hazardous electrolytes (which include cyanide-based baths and hexavalent chromium) has been encouraged by environmental restrictions. Ionic liquids, boron-free solutions, including trivalent chromium-based versions provide safer substitutes without losing effectiveness.
Electroplating remains a vital surface treatment in industrial manufacturing, enhancing part durability, corrosion resistance, and reducing maintenance. Widely used in sectors like warehousing and automotive, it continues to evolve with greener chemicals, automation, and digital integration.
At Apex Rapid, we offer high-quality electroplating services tailored for CNC-machined components. If you need reliable surface finishing, contact us today to learn how we can support your project
