
Corrosion remains one of the most persistent and costly threats to metal components—especially stainless steel—in industrial manufacturing and storage. Passivation is a controlled chemical process that enhances a metal’s natural corrosion resistance by modifying its surface chemistry, making it one of the most effective ways to protect critical parts.

The method of passivation, which frequently occurs by acid treatment, includes removing free iron along with additional impurities form the outermost layer of stainless steel or comparable alloys. A fragile, protective oxide layer which acts as an obstacle against moisture, oxygen, other corrosive substances can form or regenerate as the consequence of this process.
The strength & cleanliness of stainless-steel surface are essential for sectors like chemical storage, food processing, pharmaceuticals, & aerospace. In such instances, passivation frequently fulfills a regulatory require to satisfy ASTM, FDA, or AMS standards, as well to being a process which improves quality.
The electrochemical physics behind the passivation process, in addition to its technical procedures, standards, materials, and important significance in corrosion prevention & compliance throughout industrial sectors, are all thoroughly addressed in this article.
Fundamentally, passivation is a surface conditioning method with roots in materials science & electrochemistry. By forming a persistent, inert oxide layer on the outermost layer of the metal, it helps improve the resistance to corrosion of metals, particularly stainless steel. nevertheless must first comprehend how metal corrodes as well as how some alloys resist rust in order to fully understand how it operates.
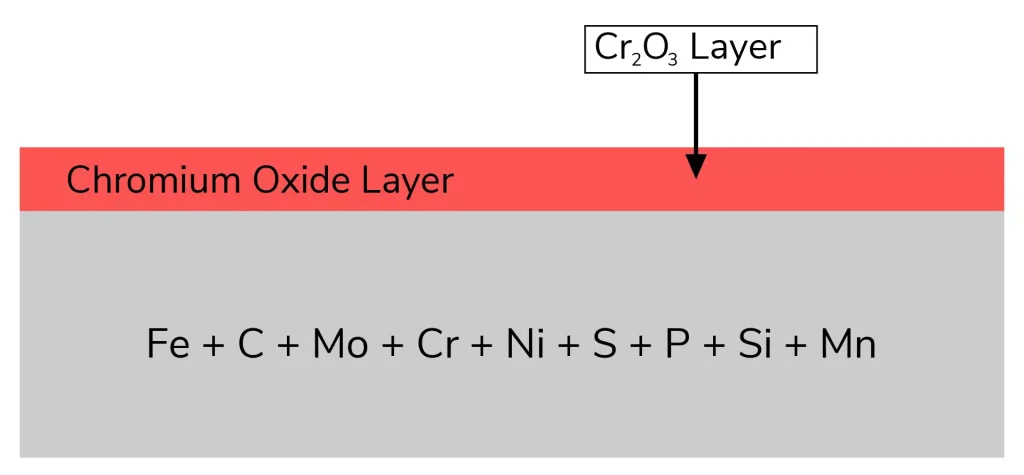
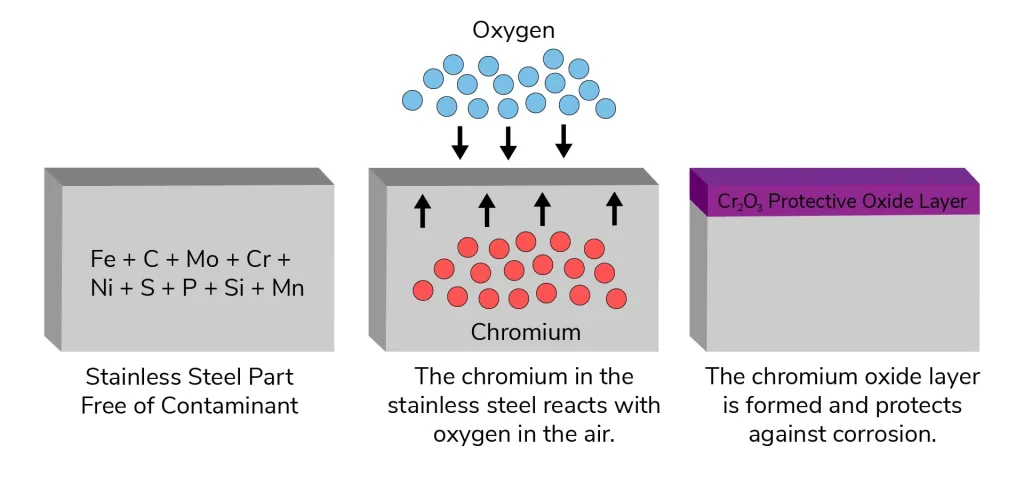
Although stainless steel is referred to as "stainless," corrosion can still occur. Its chromium content, that's usually above 10.5%, is the main factor leading to its corrosion resistance. A thin, passive coating of chromium oxide (Cr₂O₃) is formed on the outermost layer of the metal when chromium gets exposed to oxygen. This movie is:
However, loose iron or other foreign particles can enter the stainless steel's surface during manufacturing procedures like machining, welding, or shaping. These foreign substances have the ability to cause problems with the chromium oxide layer's growth, resulting in specific corrosion sites like pitted or rust bloom.
While the passive layer of stainless steel can develop naturally in air, the procedure can be inconsistent & slow, especially following mechanical processing. Biochemical passivation becomes essential at that point.
By submerging the element in an oxidizing acid, usually citric acid (C₆H₈O₇) or nitric acid (HNO₃), you may eliminate free iron & contaminants more quickly. By encouraging the establishment of a more stable and constant passive layer, this treatment fully restores the substance's resistance to corrosion.
From a scientific perspective, the process entails:
In the end, passivation is a crucial aspect of contemporary industrial corrosion prevention strategies since it goes above simply cleaning a metal to basically increase its molecular resilience against environmental attack.
In a nutshell passivation is a vital part of modern industrial corrosion management procedures since it extends beyond simply washing a metal to basically increase its molecular resistance to outside attack.
For passivation to be beneficial, a multi-step chemical treatment method needs to be executed precisely. To make sure that impurities are removed & the surface of stainless steel has been cleaned for optimal resistance to corrosion, each step is crucial. A detailed explanation of the industrial passivation process may be found below.

To get rid of oils, machining lubricants, welding oxides, & embedded particles, the process commences with mechanical or chemical cleaning. These contaminants may hinder complete acid access during passivation & result in an uneven oxide coating when they are not eliminated. Typically, cleaning takes place with.
Cleaning is finished by a thorough rinse with deionized (DI) water. It is necessary to use DI water in order to avoid mineral deposits, which might diminish the efficacy of passivation and result in water streaks on polished parts.
Parts that were recently cleaned have been immersed in a regulated acid solution, usually:
After loose iron, manganese, as well as other components are removed in this process, the alloy's chromium can react with oxygen in the environment to create a stable, protective covering of chromium oxide (Cr₂O₃).
The complete removal of any remaining acidic residue is guaranteed by a second DI water rinse. Surface etching, discoloration, or postponed deterioration under storage conditions could occur from insufficient rinsing.
Both heated chambers or filtered air that is compressed are employed for drying. For components going into storing or clean-room packaging, preventing moisture traps is crucial.
Passivation is rather than just an additional process in high-spec manufacturing setups; rather, it is a quality assurance process that is governed by strict national and international regulations. For organizations where compliance is crucial, these standards establish permitted chemistries, procedural processes, & surface cleanliness thresholds.
The most utilized industrial standard, ASTM A967, specifies permissible nitric or citric acid passivation techniques, such as:
Five methods for verification tests:
A967 is utilized extensively in the chemical, food manufacturing, & general industrial industries.
This standard offers thorough instructions for:
In the manufacturing of energy, pharmaceutical, and structural equipment, ASTM A380 is frequently cited.
AMS 2700, published by SAE International, is a must in the defense & aerospace industries. It details:
Three forms of citric and seven types of nitric acid passivation:

Although stainless steel is usually associated with passivation, it may be used on many kinds of resistant to corrosion alloys provided that they have enough chromium, nickel, or molybdenum to help with the production of passive films. But not each metal gains from this method in a comparable way.
Due to their natural chromium-rich composition, which creates a thin layer of chromium oxide (Cr₂O₃) following treatment, stainless steels are perfect for passivation. Common grades that react positively include:
Low carbon steels like 316L and 304L are particularly popular for storage applications due to their exceptional weldability & resistance to corrosion.

In numerous industrial industries where resistance to corrosion, metal purity, & regulatory compliance are unchangeable, passivation is a crucial surface treatment. The primary application areas mentioned below show how passivation extends component life & safeguards expensive equipment.
Stainless steel must be capable to withstand the rouging plus pitting corrosion brought on by high-purity water & cleaning chemicals within cleanroom settings. Passivation guarantees:
Chemical corrosion & microbiological contamination are prevented in:
Here, stainless steels are required to conform to 3-A Sanitary Standards to retain their inertness when in contact with acidic & alkaline foods.
Passivating outstanding performance metals used in hydraulic systems, missile housings, and airframes allows them to:
Passivation may be applied to downhole instruments, valves, & oil, gas, and petrochemical pipelines to:
Keep stainless components safe while being shipped & stored for an extended period of time.
Water systems' pipework & storage tanks are passivated to:
Just when the results of passivation remain the same is it effective. A component's surface is highly vulnerable to recontamination or mechanical damage following it has undergone chemical treatment in order to eliminate free iron and generate a passive oxide layer, especially during transportation and storage after the process. If passivated parts do not have protection in industrial manufacturing & storage settings, surface corrosion, early degradation, & expensive rework may result.
Interaction with carbon steel fixtures, workbenches, or tools after passivation is one of the most frequent mistakes that might result in the introduction of free iron. This weakens the passive layer & causes corrosion, which is frequently misinterpreted for passivation failure.
To preserve the structural integrity of passivated elements over time, controlled conditions are necessary:
Whilst passivation is a tried-and-true technique to improve stainless steel's resistance to corrosion, it isn't a panacea. There are a number of restrictions & boundary conditions that might render passivation ineffective or indicate other treatments might be more suitable.
Heavy oxidation, mill scale, & heat tint are not removed by passivation. To restore a clean, reactive surface in these situations, pickling and vigorous acid treatment utilizing hydrofluoric & nitric acid is necessary before passivation.
Passivation by themselves is unable to completely remove the presence of oils, embedded grinding particles, or chlorides on the surface. Whether any acid treatment to be effective, these should have been pre-cleaned with degreasers, alkaline detergents, or ultrasonic baths.
Carbon steels are unable to be passivated because they do not contain enough chromium to develop a passive oxide layer. Instead, these materials need to be plated, galvanized, or coated for protection.
Before passivation, weld seams frequently need to be pickled, electropolished, or ground. Heat-affected zones (HAZ) may not passivate uniformly & are vulnerable to chromium loss.
Alternative Surface Treatments:
| Process | Purpose | When to Use |
| Electropolishing | Micro smoothens & brightens surface | High purity & mirror-finish applications |
| Coating or Plating | Adds physical wall (zinc, epoxy, paint) | For non-passively materials like mild steel |
| Mechanical Finishing | Abrasive cleaning (e.g., blasting, tumbling) | Prepares rough or scaled surfaces |
Passivation is a critical surface treatment that improves the corrosion resistance and cleanliness of stainless steel parts, especially in demanding industries like aerospace, food, pharma, and energy. By removing free iron and forming a stable chromium oxide layer, it ensures long-term durability and compliance.
Proper pre-cleaning, adherence to standards like ASTM A967 or AMS 2700, and post-treatment care are key to its success. For more demanding applications, treatments like pickling or electropolishing may also be required.
ApexRapid offers precision passivation services for CNC-machined stainless steel parts—helping you meet strict performance and regulatory requirements.
